In 2013, a paper was published in the journal Ophthalmology, by Carl Awh, MD, and colleagues, from the group, Tennessee Retina, located in Nashville, Tennessee. In this research, the authors analyzed the data from the AREDS trial, by correlating genetic make-up to AMD progression or stability, based on consumption of certain components of the AREDS formula. In short, the authors found that certain components of the AREDS formula may either be beneficial or harmful, depending on a patient’s genotype in the CFH and ARMS2 genes.[55]
AREDS Vitamins Make Some Patients Worse and Others Better?
CFH is the acronym for Complement Factor H and ARMS2 is the acronym for Age-Related Maculopathy Susceptibility 2 gene. Both of these genes are known to play a role in the genetic susceptibility to AMD. A certain polymorphism (variant) of the CFH gene, known as CFH Y402H, is associated with an increased susceptibility to AMD and this has been confirmed in multiple populations and multiple studies.[56] [57] [58] [59] [60]
Genetic variants at the ARMS2 genetic location, appear to increase susceptibility to AMD, in general.[61] The ARMS2 genetic variant, known as A69S, is a relatively strong risk factor for AMD.[62]
However, let’s keep the risk of these two genetic variants, in perspective. Because, even in the worst-case scenario, the increased risk is not as severe as one might think. Genes are carried as alleles, which means one from each parent; we can either have none, one, or two alleles (genes) of each type. If one has two copies of the CFH Y402H allele, they would develop wet AMD 2.8 years sooner, on average, compared to those who have no copies of the gene.[63] For those who have two copies of the ARMS2 A69S gene, they develop wet AMD 5.2 years sooner than an individual with no copies of the gene, again, on average.63
So, it’s not as if, let’s say you have the worst case scenario, genetically, that you’re going to get AMD 20 years sooner – that’s just not at all the case.
Alright, so back to the genetic make-up of an individual and their risk of AMD progression, based on AREDS supplements consumption.
In 2013, Carl Awh, M.D., and colleagues, retrospectively analyzed 995 patients from the AREDS trial, who had category 3 (intermediate disease) AMD. They analyzed the outcomes of these patients, based on the genetic make-up of the CFH and ARMS2 alleles for each individual, stratified by whether the patients received antioxidants, zinc, both, or neither. They reported that patients with certain genetic profiles experienced more favorable outcomes with certain nutritional supplements, as compared to others.
In fact, Awh’s team reported that 49 percent of the subjects who received the AREDS formula were worse off than if they had received any of the other supplements. They also reported that, 13% of the group – those with high CFH and low ARMS2 risk genes, would have a more than doubling of progression of disease, if taking the AREDS supplement with zinc.[64]
In response to Awh’s research, the AREDS investigators then completed their own analysis of a subgroup of patients from the AREDS trial, for which they had genetic analyses. These 1,237 patients had either category 3 or 4 (intermediate or advanced) AMD. In this analysis, the AREDS investigators determined that there were no significant associations between genetic types and nutritional supplementation, stating, “At this stage, genetic testing does not appear helpful in improving treatment with AREDS supplements.” [65]
In 2015, Awh and colleagues then published yet another paper, analyzing some 989 patients from the AREDS trial, in patients with either category 3 or 4 AMD. Once again, they reported rather complex relationships between genetic categories, stratified based on the CFH and ARMS2 genes, and the various combinations of supplements. They indicated that, in their analysis, “most” patients would benefit from either no supplementation at all or a supplement other than the AREDS formulation, which included antioxidants and zinc.[66] By a “supplement other than the AREDS formulation,” the authors were referring to either antioxidants alone, zinc alone, or neither, given that the AREDS formula consisted of antioxidants (vitamins E and C and beta-carotene) plus zinc.
Awh’s group also argued that Dr. Chew and colleagues (who represent the AREDS study group), in dividing the study group into 27 different categories, induced a “lack of statistical power,” which resulted in data that failed “to demonstrate the benefit of the AREDS formulation for patients in any of the 27 subgroups.” (66)
If I do say so myself, I believe that Awh’s argument makes statistical sense, as some of the groups in the AREDS re-analysis had less than ten people. Out of the entire group of subjects, only 2317 had a genetic specimen, and only 882 progressed from no AMD, early or intermediate AMD, to advanced disease during the course of the trial. Each time this total number is reduced, by splitting into various categories, the statistical power of the study is markedly reduced.
In any case, in response to this second study by Awh and colleagues, the AREDS investigators, once again, attempted to replicate the analysis that Awh’s group had completed. In fact, the AREDS group identified 526 patients from the original trial, analyzed their genetics and outcomes based on the various supplement combinations, and reported that the findings by Awh et al could not be replicated. They reported that the original AREDS formulation, which consists of the antioxidants plus zinc, was the most beneficial nutritional supplement for all genetic groups analyzed.[67] The AREDS investigators advised against routine genetic testing, once again.
The American Academy of Ophthalmology weighed-in on this issue of genetic testing for AMD, in 2014, and they haven’t changed their stance since. The bottom line? They wrote, “Avoid routine genetic testing for genetically complex disorders like age-related macular degeneration…” [68]
In 2016, ophthalmologist Stephen G. Schwartz, MD, and colleagues, at Bascom Palmer Eye Institute, University of Miami, reviewed all of the evidence and in a paper published in the journal, Clinical Ophthalmology, they wrote “…at present there is not convincing evidence that genetic testing is beneficial in the routine clinical care of patients with AMD.” [69]
In 2016, professor of ophthalmology, Johanna M. Seddon, MD, and colleagues, of Tufts University School of Medicine, completed yet another analysis of the AREDS data with respect to the AREDS supplement groups. The conclusion segment of the abstract of their paper reads, very succinctly and simply, “The effectiveness of antioxidant and zinc supplementation appears to differ by genotype. Further study is needed to determine the biological basis for this interaction.” [70]
To say that there is disagreement on the issue is, obviously, quite an understatement.
Do We Give Awh’s Research the Benefit of the Doubt? Do We Genetically Test, & Then Choose a Supplement?
So maybe you’re just not convinced one way or the other. Obviously, there’s ample disagreement amongst all scientific groups, including myself.
Oh yeah, I don’t agree with either the AREDS investigators, or with Awh’s group – at least in terms of overall findings, impression, and recommendations. That’s coming up shortly.
But perhaps you’re still thinking that you want the genetic testing in order to best determine which, if any, supplements would be best? Or is it possible that no supplements would be best?
Interestingly, just to make decisions a little more difficult (or easier, depending on your perspective), in May of 2017, the Centers for Medicare and Medicaid Services (CMS) gave conditional approval for reimbursement of so-called pharmacogenetic testing for patients who have AMD. This, despite the fact that another “arm” of the government, the National Institutes of Health (NIH), which was indeed the entity behind the AREDS trial, recommended that genetic testing not be routinely conducted. But, that isn’t the end of this story.
At the time of this writing, I have been informed that, although the Centers for Medicare and Medicaid Services (CMS) has created and approved a CPT code for reimbursement of the genetic testing for AMD patients who already have the disease, the NIH has balked and the code is not currently being reimbursed.
So even if you want the genetic testing, you’ll pay for it out of your own pocket.
And as it turns out, ophthalmologist Carl Awh, MD, the lead investigator and author of the studies assessing genetics and (AREDS) supplements for AMD, is an equity owner and scientific advisory board member of ArcticDx Inc., the very company behind the genetic testing for AMD. This should not influence our consideration for the testing, but I felt it only just that I should submit for your consideration, his financial disclosure, which is required by the journals, and which is part and parcel of his scientific published papers.
Arctic Diagnostics, Genetic Testing, and Supplements
Since introducing my hypothesis regarding the cause (and treatment) of macular degeneration, in August, 2016 at the Ancestral Health Symposium – 2016, which was held at the University of Colorado Boulder, I’ve had numerous speaking engagements around the United States. For many of those speaking engagements, I’ve shared the stage with either Greg Hines, President and CEO of ArcticDx, or with Gerry Belgraver, another representative of ArcticDx. This is the case, because we’re all three regularly invited speakers for the Macular Degeneration Association (MDA) (MacularHope.org).
Quite honestly, I like both of these guys. I’ve even had lunch with Gerry Belgraver, after one of the MDA conferences. However, my hypothesis, supportive research, and treatment recommendations, are all diametrically opposed to that of ArcticDx – and these gentlemen know that. Nevertheless, I sent an email to CEO Greg Hines, and he gave me permission to share the data and tables that they present, both in their live conferences, and on their website (ArcticDx.com).
Again, we must return to the research of Carl Awh and colleagues, which, as you now know, is founded and supported by ArcticDx. In order to understand all this data regarding the genetics of AMD, with their associated supplement recommendations, we must understand a bit of the nomenclature that they use.
So this is not hard – because it’s all based on those two genes: CFH and ARMS2. Those genes, again, are the two that have by far the greatest impact on AMD risk. Well, at least as far as orthodox ophthalmology and ArcticDx is concerned. We’ll get to my position, soon.
Remember that we get two alleles (genes) from each parent – and that we can either have none, one, or two copies of each allele? So ArcticDx makes the following designation for each gene: If you have no copies of the risky CFH gene, that’s coded as “C0.” If you have one copy of the gene, that’s “C1,” and if you have two copies of the gene, that’s “C2.”
And the same goes for the ARMS2 risky gene. If you have no copies of the gene that carries the risk, that’s “A0.” If you have one copy, that’s “A1,” and if you have two copies, that is “A2.”
Got it? So, from their website, in review of some of this research, they state, “The data demonstrated that even though most patients benefitted from AREDS [supplements], for 13% of patients with a specific genetic profile (2 high-risk CFH alleles and 0 ARMS2 risk alleles) the standard AREDS formula was detrimental and accelerated vision loss much faster than placebo.” [71] The latter genetic profile, that is, 2 of the high-risk CFH alleles and none of the ARMS2 risk alleles, would be encoded as “C2A0.” Make sense?
The data from Awh and ArcticDx, based on their analysis of the AREDS study data, finds that the AREDS formula was beneficial in 69% of patients. In the other 31% of patients, the effect of the AREDS formula was actually harmful. For those with the C2A0 genetic profile, there was a 78% increased progression to advanced AMD, with associated vision loss, as compared to placebo. Here’s their table (courtesy of ArcticDx):
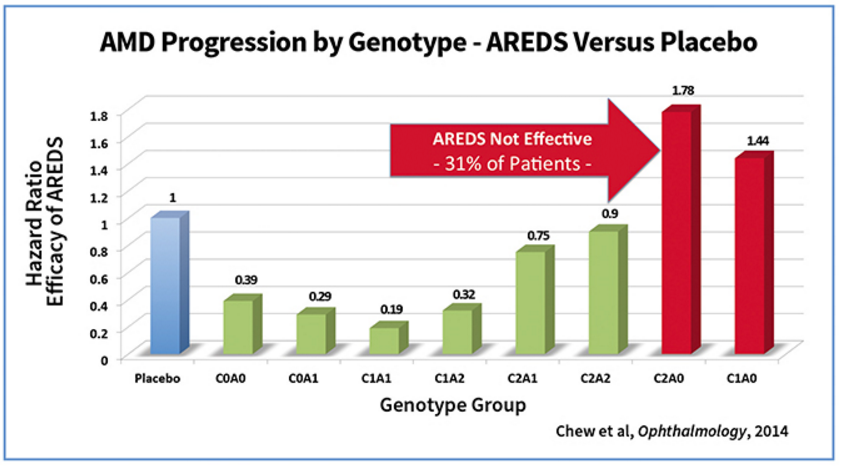
For those who are not graph/table savvy, the above data shows, for example, that those with the C1A1 genetic profile, had an AMD progression that was .19 (19%) the rate of those given placebo, versus those in the C2A0 category, who progressed at a rate 1.78 times faster than those in the placebo category.
ArcticDx did the same thing for the study cited above, by Dr. Seddon and colleagues (2016) along with that of Dr. Awh and colleagues (2015). Once again, here is their table:
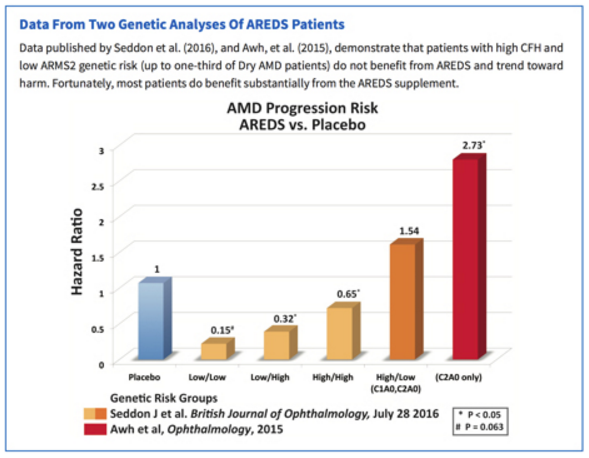
In this table, once again the designations are in reference to the CFH and ARMS2 genetic profiles, so those designated as “Low/Low” may have no CFH risk alleles and no ARMS2 risk alleles, whereas those designated as “High/Low,” as you can see, could either have the C1A0 profile or the C2A0 profile.
In this particular analysis, the “Low/Low” risk group, when given the AREDS formula, only progressed 0.15 times as fast (15%) as those given placebo. And this was statistically significant (1.0).
The conclusion of all of this, is that in the original AREDS study, we know that only 1 out of 13 (8%) patients had a beneficial result. Dr. Awh and colleagues, along with the ArcticDx team, holds that “this is a blended result for all patients – some patients who benefit and some patients who don’t.” (71)
________________________
This is Part Five of an eight-part series.
________________________
Part 1: Are the Best Vitamins for Macular Degeneration Synthetic?
Part 2: The History of Vitamins
Part 3: Vitamins and AMD
Part 4: Vitamins, AMD, and The Effects of Supplementation
Part 6: Coming soon
Part 7:Coming soon
Part 8: Coming soon

